WillD
Expert
- Joined
- Jul 19, 2021
- Messages
- 645
- Reaction score
- 913
- Points
- 93
Reaction scheme:
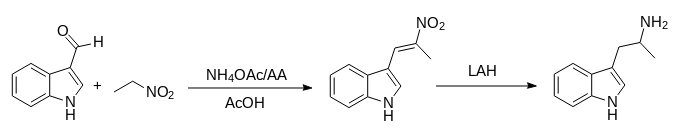
alpha-Methyl-beta-indolenideniumethyl Nitronate.
1. A mixture of 22.0 g (0.28 mole) of crystalline ammonium acetate, 6 ml acetic anhydride, and 20 ml of glacial acetic acid was stirred and warmed for approximately 20 min.
2. A mixture of 28.8 g (0.2 mole) of indole-3-aldehyde, 100 ml nitroethane, and 120 ml of glacial acetic acid was added to the solution.
3. When the mixture was brought near reflux, 14.0 g of anhydrous sodium acetate was added.
4. At reflux, 20 ml of acetic anhydride was added to the dark solution during 2 h.
5. After 2 h, the solution was allowed to cool while 45 ml of water was slowly added.
6. The solid was collected and washed with a solution of 100ml acetic acid and 45 ml of water.
7. After crystallisation from dilute alcohol, the product weighed 20.2 g (50%) and melted at 190-192 *C.
8. The analytical sample melted at 192-193 *C.
alpha-Methyltryptamine.
1. Five grams (0.024 moles) of alpha-methyl-beta-indolenideniumethyl nitronate was placed in a drip extractor
2. A mixture of 5.7 g (0.15 mole) of lithium aluminum hydride and 2000 ml of ether was stirred and refluxed for 4 hours, until all the compound was extracted into the reaction mixture.
3. The mixture was decomposed with wet ether, followed by the addition of water and then potassium hydroxide.
4. The suspension was filtered and the filtrate was dried over potassium carbonate and concentrated.
5. The residue was crystallized from ethyl acetate-petroleum ether (bp 60-71 *C) to give 2.0 gram (71%), mp 97-100 *C.
alpha-Methyl-beta-indolenideniumethyl Nitronate.
1. A mixture of 22.0 g (0.28 mole) of crystalline ammonium acetate, 6 ml acetic anhydride, and 20 ml of glacial acetic acid was stirred and warmed for approximately 20 min.
2. A mixture of 28.8 g (0.2 mole) of indole-3-aldehyde, 100 ml nitroethane, and 120 ml of glacial acetic acid was added to the solution.
3. When the mixture was brought near reflux, 14.0 g of anhydrous sodium acetate was added.
4. At reflux, 20 ml of acetic anhydride was added to the dark solution during 2 h.
5. After 2 h, the solution was allowed to cool while 45 ml of water was slowly added.
6. The solid was collected and washed with a solution of 100ml acetic acid and 45 ml of water.
7. After crystallisation from dilute alcohol, the product weighed 20.2 g (50%) and melted at 190-192 *C.
8. The analytical sample melted at 192-193 *C.
alpha-Methyltryptamine.
1. Five grams (0.024 moles) of alpha-methyl-beta-indolenideniumethyl nitronate was placed in a drip extractor
2. A mixture of 5.7 g (0.15 mole) of lithium aluminum hydride and 2000 ml of ether was stirred and refluxed for 4 hours, until all the compound was extracted into the reaction mixture.
3. The mixture was decomposed with wet ether, followed by the addition of water and then potassium hydroxide.
4. The suspension was filtered and the filtrate was dried over potassium carbonate and concentrated.
5. The residue was crystallized from ethyl acetate-petroleum ether (bp 60-71 *C) to give 2.0 gram (71%), mp 97-100 *C.
Last edited by a moderator:
