- Joined
- Aug 20, 2022
- Messages
- 2
- Reaction score
- 1
- Points
- 3
Disclaimer about this post: these synthesies are taken from patent "US2997470A" which was published in the mid 1950's
So it can be assumed that the yeilds given in the synthesies listed below can be improved by using newer methods of coupling
the amine to the D-lysergic acid Nitrogen. for example PyBOP or CDI (carbonyldiimidazole) - are both newer coupling agents
which have been used in the more recent past and might give higher yeilds than the coupling agent used in the procedures in the
patent , which are listed below. (TFAA- trifluoroacetic acid via the mixed anhydride of Lysergic acid)
-planning on updating this post as i do more research any imput or questions would be appreciated
Preperation of TFAA D-lysergic acid anhydride:
A solution containing 0.01 mol of the mixed anhydride of d-lysergic and trifiuoroacetic acids in ml. of acetonitrile is prepared as follows: 0.01 mol of d-lysergic acid is dissolved in 100 ml. of acetonitrile, and a solution of 0.021 mol of trifiuoroacetic acid anhydride in 60 ml. of of acetonitrile is added thereto. The temperature is maintained between about 20 C. to l8 C. for about one and one-half hours while the reaction is proceeding to completion.
Example 1 - Synthesis of LSB :
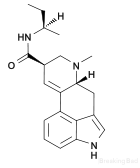
The procedure of the preperation of "TFAA D-Lysergic acid anhydride (above) is followed, With the exception that 0.001 mol of the mixed anhydride of lysergic acid and trifluoroacetic acid are treated with 0.006 mol of sec-butyl amine in place of. A residue comprising d-lysergic acid N-secbutyl amide and d-isolysergic acid N-sec-butyl amide results after evaporation of the chloroform extract to dryness. The maleate salt of d-lyser gic acid N-secbutyl amide is prepared by dissolving the residue in methanol, adding maleic acid and then ether. Fine needles of crystalline d-lysergic acid N-sec-butyl amide acid maleate precipitate immediately. About 30 mg. of crystalline dlysergic acid N-sec-butyl amide acid maleate having a decomposition point of about 216 C. are obtained.
Analysis.-Calculated for C H N O C, 65.43; H, 6.65; N, 9.56. Found: C, 65.29; H, 6.71; N, 9.53.
The mother liquors from the isolation of the d-lysergic acid N-sec-butyl amide acid maleate are evaporated to dryness and the d-isolysergic acid N-sec-butyl amide acid maleate therein is converted to the corresponding free base by the method disclosed in Example 1. The free base is chromatographed over basic alumina using a 4:1 benzene-chloroform mixture for elution. d--Isolysergic acid N-sec-butyl amide is eluted and the residue remaining after evaporation of the solvent from the eluate, is crystallized from benzene yielding d-isolysergic acid N sec-butyl amide, melting with decomposition at about -191 C.
Example 2 - Synthesis of LSM:
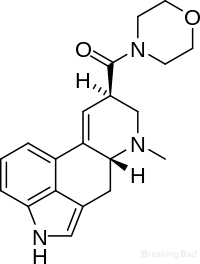
A solution containing 0.02 mol of the mixed anhydride of d-lysergic acid and trifluoroacetic acid is prepared as follows: A suspension of 0.02 mol of d-lysergic acid in 225 ml. of acetonitrile previously cooled to -20 C. is mixed with a solution of 0.042 mol of trifluoroacetic acid anhydride in 100 ml. of acetonitrile also previously cooled to 20 C., and 0.2 mol of finely divided magnesium oxide are added. The reaction mixture is kept at 20 C. for about one and one-half hours, whereupon the suspended d lysergic acid dissolves and the mixed anhydride of lysergic acid and trifluoroacetic acid is formed.
The mixed anhydride solution is then added to 0.104 mol of morpholine dissolved in 150 ml. of acetonitrile maintained at room temperature. The reaction mixture is allowed to stand for one and one-fourth hours in the dark. The solvents are thereafter evaporated in vacuo and the resulting residue, comprising lysergic acid morpholide, is dissolved in 400 ml. of chloroform. The chloroform solution is washed twelve times with 50 ml. portions of water, dried over anhydrous sodium sulfate and the chloroform is evaporated in vacuo. The resulting dark brown residue is dissolved in 20 ml. of anhydrous methanol and a solution containing 1.2 g. of maleic acid in 5 ml. of methanol is added thereto, whereupon the maleate salt of the d-lysergic acid morpholide is formed. Twentyfive milliliters of anhydrous diethyl ether are then added Analysis.-Calculated for C H N O C, 63.56; H, 6.00; N, 9.27. Found: C, 63.22; H, 5.75; N, 9.04.
The maleate salt is dissolved in a minimum volume of water and neutralized with sodium carbonate. The resulting mixture is extracted with three ml. portions of chloroform, the combined chloroform extracts are dried with sodium sulfate, are filtered, and are evaporated to dryness in vacuo. The residue is taken up in a small volume of benzene and cooled, whereupon crystals of d-lysergic acid morpholide are formed.
The combined filtrate and washings from the isolation of d-lysergic acid morpholide acid maleate are evaporated to dryness. The acid maleate salt of d-isolysergic acid morpholide contained in the residue is dissolved in water, is converted to the corresponding free base by treatment with sodium carbonate and is extracted with chloroform as set forth hereinabove. The chloroform solution containing the free base is evaporated to small volume, is placed on a column of 50 g. of basic alumina and is chromatographed using a 1:1 benzene-chloroform mixture as the eluant. The fractions of the eluate giving a positive Van Urk test are combined and are evaporated to dryness. Crystallization of the resulting residue from acetone yields about 550 mg. of d-isolysergic acid morpholide melting at about 199-200 C. with decomposition.
Example 3 - Synthesis of MiPLA:
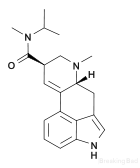
A solution of 0.02 mol of the mixed anhydride of dlysergic acid and trifluoroacetic acid in 225 ml. of acetonitrile is prepared according to the procedure of "Preperation of TFAA D-lysergic acid anhydride" at the top of the post. This solution is added to 0.104 mol of methyl isopropyl amine dissolved in 200 ml. of acetonitrile, and the mixture is allowed to stand in the dark for about one hour and then is evaporated to dryness. The residue is taken up in about 400 ml. of chloroform and the solution is washed with ten successive 50 ml. portions of water. The washed chloroform solution is dried with anhydrous magnesium sulfate and is evaporated to dryness. The residue comprises a mixture of both the normal and iso d-lysergic acid N-methyl N-isopropyl amides. A solution of 3.5 g. of the amide mixture in about 100 ml. of a 1:9 chloroform-benzene solvent mixture is placed on a chromatographic adsorption column containing about 110 g. of basic alumina. The first liter of eluate, using the same solvent mixture, after evaporation of the eluting solvent and recrystallization of the resulting residue from ethyl acetate, yields about 340 mg. of d-lysergic acid N-methyl-N-isopropyl amide, melting at about 196- 197 C. with decomposition. The chromatogram is further developed with another one liter portion of the same eluent followed by 100 ml. of pure chloroform, and these eluates comprise mixtures of the normal and iso amides. Further elution with 200 ml. of pure chloroform results in an eluate which yields a solid residue of d-isolysergic acid N-methyl N-isopropyl amide after evaporation of the eluting solvent. Recrystallization of this residue from ethyl acetate yields about 860 mg. of d-isolysergic acid N-mcthy1 N-isopropyl amide melting at about 194-195 C. with decomposition.
Analysis.Calculated for C H N O: C, 74.25; H, 7.79; N, 12.99. Found: C, 73.85; H, 7.86; N, 12.78.
d-Lysergic acid N-methyl N-n-propyl amide is prepared as set forth above using methyl n-propyl amine in place of methyl isopropyl amine. It is purified in the form of its acid ta-rtrate salt as set forth in Example 9. d-Lysergic acid N-methyl N-n-propyl amide acid tartrate melts at about 160 C. with decomposition.
d-Isolysergic acid N-methyl N-n-propyl amide is also purified by chromatography. It melts at about 180- 181 C. with decomposition.
Analysis=-Calculated for C I-I N O: C, 74.25; H, 7.79; N, 12.99. Found: C, 74.24; H, 8.00; N, 12.76.
So it can be assumed that the yeilds given in the synthesies listed below can be improved by using newer methods of coupling
the amine to the D-lysergic acid Nitrogen. for example PyBOP or CDI (carbonyldiimidazole) - are both newer coupling agents
which have been used in the more recent past and might give higher yeilds than the coupling agent used in the procedures in the
patent , which are listed below. (TFAA- trifluoroacetic acid via the mixed anhydride of Lysergic acid)
-planning on updating this post as i do more research any imput or questions would be appreciated
Preperation of TFAA D-lysergic acid anhydride:
A solution containing 0.01 mol of the mixed anhydride of d-lysergic and trifiuoroacetic acids in ml. of acetonitrile is prepared as follows: 0.01 mol of d-lysergic acid is dissolved in 100 ml. of acetonitrile, and a solution of 0.021 mol of trifiuoroacetic acid anhydride in 60 ml. of of acetonitrile is added thereto. The temperature is maintained between about 20 C. to l8 C. for about one and one-half hours while the reaction is proceeding to completion.
Example 1 - Synthesis of LSB :
The procedure of the preperation of "TFAA D-Lysergic acid anhydride (above) is followed, With the exception that 0.001 mol of the mixed anhydride of lysergic acid and trifluoroacetic acid are treated with 0.006 mol of sec-butyl amine in place of. A residue comprising d-lysergic acid N-secbutyl amide and d-isolysergic acid N-sec-butyl amide results after evaporation of the chloroform extract to dryness. The maleate salt of d-lyser gic acid N-secbutyl amide is prepared by dissolving the residue in methanol, adding maleic acid and then ether. Fine needles of crystalline d-lysergic acid N-sec-butyl amide acid maleate precipitate immediately. About 30 mg. of crystalline dlysergic acid N-sec-butyl amide acid maleate having a decomposition point of about 216 C. are obtained.
Analysis.-Calculated for C H N O C, 65.43; H, 6.65; N, 9.56. Found: C, 65.29; H, 6.71; N, 9.53.
The mother liquors from the isolation of the d-lysergic acid N-sec-butyl amide acid maleate are evaporated to dryness and the d-isolysergic acid N-sec-butyl amide acid maleate therein is converted to the corresponding free base by the method disclosed in Example 1. The free base is chromatographed over basic alumina using a 4:1 benzene-chloroform mixture for elution. d--Isolysergic acid N-sec-butyl amide is eluted and the residue remaining after evaporation of the solvent from the eluate, is crystallized from benzene yielding d-isolysergic acid N sec-butyl amide, melting with decomposition at about -191 C.
Example 2 - Synthesis of LSM:
A solution containing 0.02 mol of the mixed anhydride of d-lysergic acid and trifluoroacetic acid is prepared as follows: A suspension of 0.02 mol of d-lysergic acid in 225 ml. of acetonitrile previously cooled to -20 C. is mixed with a solution of 0.042 mol of trifluoroacetic acid anhydride in 100 ml. of acetonitrile also previously cooled to 20 C., and 0.2 mol of finely divided magnesium oxide are added. The reaction mixture is kept at 20 C. for about one and one-half hours, whereupon the suspended d lysergic acid dissolves and the mixed anhydride of lysergic acid and trifluoroacetic acid is formed.
The mixed anhydride solution is then added to 0.104 mol of morpholine dissolved in 150 ml. of acetonitrile maintained at room temperature. The reaction mixture is allowed to stand for one and one-fourth hours in the dark. The solvents are thereafter evaporated in vacuo and the resulting residue, comprising lysergic acid morpholide, is dissolved in 400 ml. of chloroform. The chloroform solution is washed twelve times with 50 ml. portions of water, dried over anhydrous sodium sulfate and the chloroform is evaporated in vacuo. The resulting dark brown residue is dissolved in 20 ml. of anhydrous methanol and a solution containing 1.2 g. of maleic acid in 5 ml. of methanol is added thereto, whereupon the maleate salt of the d-lysergic acid morpholide is formed. Twentyfive milliliters of anhydrous diethyl ether are then added Analysis.-Calculated for C H N O C, 63.56; H, 6.00; N, 9.27. Found: C, 63.22; H, 5.75; N, 9.04.
The maleate salt is dissolved in a minimum volume of water and neutralized with sodium carbonate. The resulting mixture is extracted with three ml. portions of chloroform, the combined chloroform extracts are dried with sodium sulfate, are filtered, and are evaporated to dryness in vacuo. The residue is taken up in a small volume of benzene and cooled, whereupon crystals of d-lysergic acid morpholide are formed.
The combined filtrate and washings from the isolation of d-lysergic acid morpholide acid maleate are evaporated to dryness. The acid maleate salt of d-isolysergic acid morpholide contained in the residue is dissolved in water, is converted to the corresponding free base by treatment with sodium carbonate and is extracted with chloroform as set forth hereinabove. The chloroform solution containing the free base is evaporated to small volume, is placed on a column of 50 g. of basic alumina and is chromatographed using a 1:1 benzene-chloroform mixture as the eluant. The fractions of the eluate giving a positive Van Urk test are combined and are evaporated to dryness. Crystallization of the resulting residue from acetone yields about 550 mg. of d-isolysergic acid morpholide melting at about 199-200 C. with decomposition.
Example 3 - Synthesis of MiPLA:
A solution of 0.02 mol of the mixed anhydride of dlysergic acid and trifluoroacetic acid in 225 ml. of acetonitrile is prepared according to the procedure of "Preperation of TFAA D-lysergic acid anhydride" at the top of the post. This solution is added to 0.104 mol of methyl isopropyl amine dissolved in 200 ml. of acetonitrile, and the mixture is allowed to stand in the dark for about one hour and then is evaporated to dryness. The residue is taken up in about 400 ml. of chloroform and the solution is washed with ten successive 50 ml. portions of water. The washed chloroform solution is dried with anhydrous magnesium sulfate and is evaporated to dryness. The residue comprises a mixture of both the normal and iso d-lysergic acid N-methyl N-isopropyl amides. A solution of 3.5 g. of the amide mixture in about 100 ml. of a 1:9 chloroform-benzene solvent mixture is placed on a chromatographic adsorption column containing about 110 g. of basic alumina. The first liter of eluate, using the same solvent mixture, after evaporation of the eluting solvent and recrystallization of the resulting residue from ethyl acetate, yields about 340 mg. of d-lysergic acid N-methyl-N-isopropyl amide, melting at about 196- 197 C. with decomposition. The chromatogram is further developed with another one liter portion of the same eluent followed by 100 ml. of pure chloroform, and these eluates comprise mixtures of the normal and iso amides. Further elution with 200 ml. of pure chloroform results in an eluate which yields a solid residue of d-isolysergic acid N-methyl N-isopropyl amide after evaporation of the eluting solvent. Recrystallization of this residue from ethyl acetate yields about 860 mg. of d-isolysergic acid N-mcthy1 N-isopropyl amide melting at about 194-195 C. with decomposition.
Analysis.Calculated for C H N O: C, 74.25; H, 7.79; N, 12.99. Found: C, 73.85; H, 7.86; N, 12.78.
d-Lysergic acid N-methyl N-n-propyl amide is prepared as set forth above using methyl n-propyl amine in place of methyl isopropyl amine. It is purified in the form of its acid ta-rtrate salt as set forth in Example 9. d-Lysergic acid N-methyl N-n-propyl amide acid tartrate melts at about 160 C. with decomposition.
d-Isolysergic acid N-methyl N-n-propyl amide is also purified by chromatography. It melts at about 180- 181 C. with decomposition.
Analysis=-Calculated for C I-I N O: C, 74.25; H, 7.79; N, 12.99. Found: C, 74.24; H, 8.00; N, 12.76.
Last edited:
